Molecule of the Month February 2016: Captopril
 One
of the most fascinating things about my sabbatical leave at the
Liverpool School of Tropical Medicine a few years ago, was visiting the Hepetarium, which houses the Alastair Reed Snake Venom Research Unit headed up by Rob Harrison.
The power of research into toxins, which has played a major role in the
history of Biochemistry, Physiology and Medicine, in my view is often
undervalued. But not in respect of the molecule of this month,
captopril, the molecule that was introduced by the drug company Bristol
Myers Squibb (then ER Squibb) as the first ACE inhibitor for the relief
of hypertension.
One
of the most fascinating things about my sabbatical leave at the
Liverpool School of Tropical Medicine a few years ago, was visiting the Hepetarium, which houses the Alastair Reed Snake Venom Research Unit headed up by Rob Harrison.
The power of research into toxins, which has played a major role in the
history of Biochemistry, Physiology and Medicine, in my view is often
undervalued. But not in respect of the molecule of this month,
captopril, the molecule that was introduced by the drug company Bristol
Myers Squibb (then ER Squibb) as the first ACE inhibitor for the relief
of hypertension.
 The structure of captopril is shown on the right, and it is know to act on angiotensin
converting enzyme (ACE). The development of captopril, is a lovely
example of how natural products can inform drug discovery. It is a
result of a combination of Biological serendipity and incisive
Chemistry. The original molecules associated with blood pressure
modulation, extracted from venom, where too complex to be viable
therapeutically. However, by first establishing the key role of the
proline moiety in active site inhibition, the next phase was aimed at
simplifying the peptidyl arm of the molecule.
The structure of captopril is shown on the right, and it is know to act on angiotensin
converting enzyme (ACE). The development of captopril, is a lovely
example of how natural products can inform drug discovery. It is a
result of a combination of Biological serendipity and incisive
Chemistry. The original molecules associated with blood pressure
modulation, extracted from venom, where too complex to be viable
therapeutically. However, by first establishing the key role of the
proline moiety in active site inhibition, the next phase was aimed at
simplifying the peptidyl arm of the molecule.
 The natural products associated with blood pressure modulating activity were peptides of the bradykinin family (brady-slow, kinin-movement
related), including the nonapeptide, teprotide (LHS). As you can see
from the final form of the drug, the enzyme anchor can be dramatically
simplified, making the drug a real economic option. [As opposed to a
synthetic nightmare!]. The systematic evaluation of variations on the
natural product (structure-activity-relationships) led to captopril, as
the first drug of this class.
The natural products associated with blood pressure modulating activity were peptides of the bradykinin family (brady-slow, kinin-movement
related), including the nonapeptide, teprotide (LHS). As you can see
from the final form of the drug, the enzyme anchor can be dramatically
simplified, making the drug a real economic option. [As opposed to a
synthetic nightmare!]. The systematic evaluation of variations on the
natural product (structure-activity-relationships) led to captopril, as
the first drug of this class.
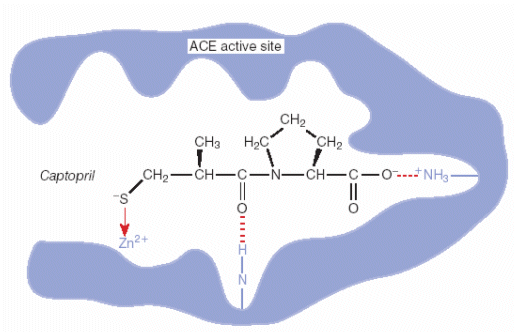 The
simple active site inhibition model is shown schematically below.
However, in order to address the many early reports of severe side
effects, further refinement has led to a new generation of ACE
inhibitors that include enalapril. There is a freely available summary
of current ACE inhibitors here.
The captopril story is an excellent example of how medicinal chemistry
developed in the 1970s in particular. It was followed by approaches
based on screening large automated libraries of compounds in a
methodology called combinatorial chemistry.
I note with interest and enthusiasm, the resurgence of Natural Product
chemistry in the areas of antibiotics and anti-malarial drugs, and
direct you to a nice review of this "renaissance" by Tadesz Molinski,
which is free to download here.
The
simple active site inhibition model is shown schematically below.
However, in order to address the many early reports of severe side
effects, further refinement has led to a new generation of ACE
inhibitors that include enalapril. There is a freely available summary
of current ACE inhibitors here.
The captopril story is an excellent example of how medicinal chemistry
developed in the 1970s in particular. It was followed by approaches
based on screening large automated libraries of compounds in a
methodology called combinatorial chemistry.
I note with interest and enthusiasm, the resurgence of Natural Product
chemistry in the areas of antibiotics and anti-malarial drugs, and
direct you to a nice review of this "renaissance" by Tadesz Molinski,
which is free to download here.
 One
of the most fascinating things about my sabbatical leave at the
Liverpool School of Tropical Medicine a few years ago, was visiting the Hepetarium, which houses the Alastair Reed Snake Venom Research Unit headed up by Rob Harrison.
The power of research into toxins, which has played a major role in the
history of Biochemistry, Physiology and Medicine, in my view is often
undervalued. But not in respect of the molecule of this month,
captopril, the molecule that was introduced by the drug company Bristol
Myers Squibb (then ER Squibb) as the first ACE inhibitor for the relief
of hypertension.
One
of the most fascinating things about my sabbatical leave at the
Liverpool School of Tropical Medicine a few years ago, was visiting the Hepetarium, which houses the Alastair Reed Snake Venom Research Unit headed up by Rob Harrison.
The power of research into toxins, which has played a major role in the
history of Biochemistry, Physiology and Medicine, in my view is often
undervalued. But not in respect of the molecule of this month,
captopril, the molecule that was introduced by the drug company Bristol
Myers Squibb (then ER Squibb) as the first ACE inhibitor for the relief
of hypertension.  The structure of captopril is shown on the right, and it is know to act on angiotensin
converting enzyme (ACE). The development of captopril, is a lovely
example of how natural products can inform drug discovery. It is a
result of a combination of Biological serendipity and incisive
Chemistry. The original molecules associated with blood pressure
modulation, extracted from venom, where too complex to be viable
therapeutically. However, by first establishing the key role of the
proline moiety in active site inhibition, the next phase was aimed at
simplifying the peptidyl arm of the molecule.
The structure of captopril is shown on the right, and it is know to act on angiotensin
converting enzyme (ACE). The development of captopril, is a lovely
example of how natural products can inform drug discovery. It is a
result of a combination of Biological serendipity and incisive
Chemistry. The original molecules associated with blood pressure
modulation, extracted from venom, where too complex to be viable
therapeutically. However, by first establishing the key role of the
proline moiety in active site inhibition, the next phase was aimed at
simplifying the peptidyl arm of the molecule. The natural products associated with blood pressure modulating activity were peptides of the bradykinin family (brady-slow, kinin-movement
related), including the nonapeptide, teprotide (LHS). As you can see
from the final form of the drug, the enzyme anchor can be dramatically
simplified, making the drug a real economic option. [As opposed to a
synthetic nightmare!]. The systematic evaluation of variations on the
natural product (structure-activity-relationships) led to captopril, as
the first drug of this class.
The natural products associated with blood pressure modulating activity were peptides of the bradykinin family (brady-slow, kinin-movement
related), including the nonapeptide, teprotide (LHS). As you can see
from the final form of the drug, the enzyme anchor can be dramatically
simplified, making the drug a real economic option. [As opposed to a
synthetic nightmare!]. The systematic evaluation of variations on the
natural product (structure-activity-relationships) led to captopril, as
the first drug of this class.  The
simple active site inhibition model is shown schematically below.
However, in order to address the many early reports of severe side
effects, further refinement has led to a new generation of ACE
inhibitors that include enalapril. There is a freely available summary
of current ACE inhibitors here.
The captopril story is an excellent example of how medicinal chemistry
developed in the 1970s in particular. It was followed by approaches
based on screening large automated libraries of compounds in a
methodology called combinatorial chemistry.
I note with interest and enthusiasm, the resurgence of Natural Product
chemistry in the areas of antibiotics and anti-malarial drugs, and
direct you to a nice review of this "renaissance" by Tadesz Molinski,
which is free to download here.
The
simple active site inhibition model is shown schematically below.
However, in order to address the many early reports of severe side
effects, further refinement has led to a new generation of ACE
inhibitors that include enalapril. There is a freely available summary
of current ACE inhibitors here.
The captopril story is an excellent example of how medicinal chemistry
developed in the 1970s in particular. It was followed by approaches
based on screening large automated libraries of compounds in a
methodology called combinatorial chemistry.
I note with interest and enthusiasm, the resurgence of Natural Product
chemistry in the areas of antibiotics and anti-malarial drugs, and
direct you to a nice review of this "renaissance" by Tadesz Molinski,
which is free to download here.
Influence of captopril on length of mice cardiac fibroblast telomere might be helpful in such research.
ReplyDelete