 This month I have taken the liberty of discussing a collection of related proteins, that together make up the virus at the heart of Acquired Immunodeficiency Syndrome (AIDS): the Human Immunodeficiency Virus (HIV). For the purposes of the Blog, I will consider HIV as a multi-protein complex in which an outer shell of proteins, protects an inner core that contains the RNA genome and accessory molecules. A little Biology first, HIV is a retrovirus, that is, its genome is RNA based and therefore it requires a conversion step in which the enzyme Reverse Transcriptase copies the viral genome into DNA, as a prelude to its insertion into the (human) host genome.
This month I have taken the liberty of discussing a collection of related proteins, that together make up the virus at the heart of Acquired Immunodeficiency Syndrome (AIDS): the Human Immunodeficiency Virus (HIV). For the purposes of the Blog, I will consider HIV as a multi-protein complex in which an outer shell of proteins, protects an inner core that contains the RNA genome and accessory molecules. A little Biology first, HIV is a retrovirus, that is, its genome is RNA based and therefore it requires a conversion step in which the enzyme Reverse Transcriptase copies the viral genome into DNA, as a prelude to its insertion into the (human) host genome.  The virus genome comprises 3 primary genes: Gag, Pol and Env. These 3 genes each encode a poly protein which is then processed to form the HIV proteome. The Gag proteins (for group specific antigen) are proteins that organise the interior of the virion. The Pol polyprotein encodes enzymes for replication of the genome, including reverse transcriptase, that catalyses DNA synthesis from RNA, and an Integrase enzyme that inserts the DNA into the host genome. The other key protein that is co translated with RT, is the HIV protease, itself required for processing of the polyproteins. Finally, the Env polyprotein comprises the envelope(or surface) proteins, gp120 and gp41 (gp means glycoprotein, a protein that is normally modified by the addition of carbohydrate after synthesis on the ribosome). The genome also encodes essential regulatory elements which are expressed as RNA species: these are called Tat and Rev. A good introduction on the complete set of HIV elements can be found at this link. I shall mainly focus here on some features of the proteins that have been of interest in both vaccine development and drug strategies.
The virus genome comprises 3 primary genes: Gag, Pol and Env. These 3 genes each encode a poly protein which is then processed to form the HIV proteome. The Gag proteins (for group specific antigen) are proteins that organise the interior of the virion. The Pol polyprotein encodes enzymes for replication of the genome, including reverse transcriptase, that catalyses DNA synthesis from RNA, and an Integrase enzyme that inserts the DNA into the host genome. The other key protein that is co translated with RT, is the HIV protease, itself required for processing of the polyproteins. Finally, the Env polyprotein comprises the envelope(or surface) proteins, gp120 and gp41 (gp means glycoprotein, a protein that is normally modified by the addition of carbohydrate after synthesis on the ribosome). The genome also encodes essential regulatory elements which are expressed as RNA species: these are called Tat and Rev. A good introduction on the complete set of HIV elements can be found at this link. I shall mainly focus here on some features of the proteins that have been of interest in both vaccine development and drug strategies. Reverse Transcriptase received a mention in an earlier Molecule of the Month: RNA Polymerase. This enzyme, like all nucleic acid polymerases takes the building blocks of nucleic acids, ATP, CTP etc for RNA or dATP, dCTP etc. for DNA and inserts them one by one in a single direction, as directed by the complementary sequence of bases on the template DNA (or RNA). RT carries out the opposite reaction to a typical RNA polymerase: the enzyme synthesises DNA from an RNA template. The single strand is then copied into a double stranded form and the resultant duplex is incorporated (or integrated) into the host genome by an enzyme called an Integrase. These two reactions are distinctive. Whilst there are many similarities to host nucleic acid polymerases, there are also significant mechanistic differences. This presents an opportunity for drug development. (Students, think about this concept: it is an important aspect in the design of new drugs such as antibiotics and anticancer agents). The first drug released to combat AIDS was called azidothymidine or AZT for short (its commercial name was first zidovudine and finally Retrovir; it is pictured top right). It is an inhibitor of RT, with around 100-fold preference for RT over our own DNA Polymerases. First the cell converts AZT into the triphosphate and then the RT is blocked competitively at the active site. High doses have significant side effects which are directed at the mitochondrial DNA replication machinery (causing muscle fatigue). However, it is currently used in combination with other drugs as willbe discussed below. Nevertheless, the mechanism of action of compounds such as AZT, reveals how drugs must be developed not only in respect of the target, but e so called "off target" sites, that are the source of some side effects. If you are interested, you can read about the "rise and fall of AZT", the first anti AIDS drug here.
Reverse Transcriptase received a mention in an earlier Molecule of the Month: RNA Polymerase. This enzyme, like all nucleic acid polymerases takes the building blocks of nucleic acids, ATP, CTP etc for RNA or dATP, dCTP etc. for DNA and inserts them one by one in a single direction, as directed by the complementary sequence of bases on the template DNA (or RNA). RT carries out the opposite reaction to a typical RNA polymerase: the enzyme synthesises DNA from an RNA template. The single strand is then copied into a double stranded form and the resultant duplex is incorporated (or integrated) into the host genome by an enzyme called an Integrase. These two reactions are distinctive. Whilst there are many similarities to host nucleic acid polymerases, there are also significant mechanistic differences. This presents an opportunity for drug development. (Students, think about this concept: it is an important aspect in the design of new drugs such as antibiotics and anticancer agents). The first drug released to combat AIDS was called azidothymidine or AZT for short (its commercial name was first zidovudine and finally Retrovir; it is pictured top right). It is an inhibitor of RT, with around 100-fold preference for RT over our own DNA Polymerases. First the cell converts AZT into the triphosphate and then the RT is blocked competitively at the active site. High doses have significant side effects which are directed at the mitochondrial DNA replication machinery (causing muscle fatigue). However, it is currently used in combination with other drugs as willbe discussed below. Nevertheless, the mechanism of action of compounds such as AZT, reveals how drugs must be developed not only in respect of the target, but e so called "off target" sites, that are the source of some side effects. If you are interested, you can read about the "rise and fall of AZT", the first anti AIDS drug here. 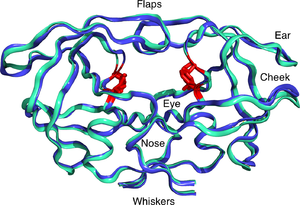 The second classical target for HIV therapy is the HIV protease (shown left), which is required for the maturation of the polyproteins expressed from the RNA genome of HIV. This enzyme uses a pair of catalytic aspartate residues, one form each chain of this dimeric enzyme, to process the HIV polyproteins. (It is sometimes called an aspartic protease). By synthesising peptide based analogues of the substrate, constrained to mimic the "transition state" of the reaction, it has become possible to treat AIDS through administration of compounds such as Ritonavir and Lopinavir. However, it is more usual to combine RT and Protease inhibitors in a cocktail to produce what are referred to as combination therapies, discussed in more detail here. This reduces the emergence of resistance, caused in part by the high mutation rates of the HIV viral genome, together with the heterogeneity of virus strains that seem to be associated with some infections. The development of anti AIDS drugs, in particular the anti-protease inhibitors, is often cited as one of the most significant successes of rational drug design, based on crystallography and modelling.
The second classical target for HIV therapy is the HIV protease (shown left), which is required for the maturation of the polyproteins expressed from the RNA genome of HIV. This enzyme uses a pair of catalytic aspartate residues, one form each chain of this dimeric enzyme, to process the HIV polyproteins. (It is sometimes called an aspartic protease). By synthesising peptide based analogues of the substrate, constrained to mimic the "transition state" of the reaction, it has become possible to treat AIDS through administration of compounds such as Ritonavir and Lopinavir. However, it is more usual to combine RT and Protease inhibitors in a cocktail to produce what are referred to as combination therapies, discussed in more detail here. This reduces the emergence of resistance, caused in part by the high mutation rates of the HIV viral genome, together with the heterogeneity of virus strains that seem to be associated with some infections. The development of anti AIDS drugs, in particular the anti-protease inhibitors, is often cited as one of the most significant successes of rational drug design, based on crystallography and modelling.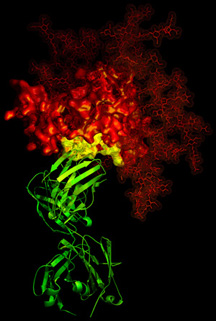 The final structure I wish to mention is the gp120 molecule, which is the target of a number of immunotherapy strategies. Again, mutation rates present a challenge for ani vaccine programme, but AIDS vaccines are particularly difficult to develop owing to the mutability of the viral genome. Professor Dennis Burton's group at Scripps in La Jolla, Callifornia, are in the vanguard of approaches to neutralise HIV. The structure on the right, from the Wilson-Burton groups at Scripps, provides detailed structural interaction information, which is currently reducing the odds of finding a potent vaccine. Indeed in last year's Science the team along with International collaborators made significant advances towards this goal. One of the most important issues that AIDS research has told us is that the recognition between proteins in the design of vaccines, must also consider the significant role played by carbohydrates and the masking of amino acids by sugars, which presents another major challenge to vaccine discovery. However, as the structural information becomes richer, success will surely come.
The final structure I wish to mention is the gp120 molecule, which is the target of a number of immunotherapy strategies. Again, mutation rates present a challenge for ani vaccine programme, but AIDS vaccines are particularly difficult to develop owing to the mutability of the viral genome. Professor Dennis Burton's group at Scripps in La Jolla, Callifornia, are in the vanguard of approaches to neutralise HIV. The structure on the right, from the Wilson-Burton groups at Scripps, provides detailed structural interaction information, which is currently reducing the odds of finding a potent vaccine. Indeed in last year's Science the team along with International collaborators made significant advances towards this goal. One of the most important issues that AIDS research has told us is that the recognition between proteins in the design of vaccines, must also consider the significant role played by carbohydrates and the masking of amino acids by sugars, which presents another major challenge to vaccine discovery. However, as the structural information becomes richer, success will surely come.Finally, the challenges to human health brought by retroiviruses such as HIV and Ebola, demonstrates how Science thrives in adversity and I am sure that despite the current Health challenges, we will be in a better position to meet new diseases that are undoubtedly lurking around the corner!
No comments:
Post a Comment