I changed my mind at the last minute over the molecule to send you on your way over the summer. I had intended to wax lyrical over the carbohydrates that decorate the surfaces of many bacteria, but I'll save that for a later Blog. Instead I though we all needed an injection of post examination energy, and what better than the ATP synthesising machinery exemplified on the left by a bacterial example. This enzyme is responsible for converting ADP (adenosine diphosphate) into the energy currency of the cell, ATP (or adenosine triphosphate). As an undergraduate, I was taught the alternative models that were current to explain the production of ATP in the cell, but the final explanation was somewhat unexpected and a triumph for one man in particular (Peter Mitchell), who stubbornly refused to be deflected from his unpopular ideas on the mechanism of ATP production.

Peter Mitchell graduated from Cambridge over 60 years ago: in fact his first project proved unsatisfactory, but in 1950 he finally earned his PhD stripes. ATP was first discovered in 1929, and in the period 1940 to 1960 the compound was synthesised chemically by Todd; a few years earlier, Lipmann had recognised its potential for harnessing biochemical energy, but despite a considerable effort attempts to rationalise the data, the evidence for its biological synthesis remained unsatisfactory. From the insights of a great British Biochemist RJP Williams, however, Mitchell was able to propose how harnessing the energy in a proton gradient, established acoss biological membranes, could be coupled to the synthesis of ATP. That is, a proton-motive-force drives structural changes in the ATP synthase: this was corroborated by the work of the famous enzymologist Paul Boyer from Los Angeles and our own Sir John Walker (below left, at Cambridge).
The union of ADP and inorganic phosphate is brought about (against an adverse energy barrier) through the coupling of a conformational change in a subunit of the ATP synthase and the movement of protons across a membrane. At the time, this idea was radical, so much so that Mitchell was forced to build his own private research facility on Bodmin Moore (a favourite location for Gothic mysteries, and the legendary home of King Arthur and the sword excalibur). The reclusive Mitchell (along with his brother) resorted to publishing his own ideas. First, it was published as a pamphlet, but two years later, he followed up with several small volumes called “little grey books” named after their rather unexciting covers. Just like change, investigative research is constant, and in the early 1970s, his theory gained significant acceptance among his peers and the scientific community in general. Meanwhile, John Walker was developing the exacting purification methods in order to obtain the mg quantities necessary for detailed structural studies:. With a structure in hand predictions can be tested, although the scale of this task was a major challenge and the confirmation came slowly.
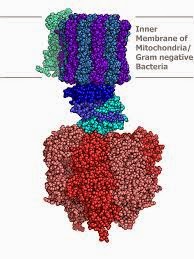
 The work of John Walker's lab at the Laboratory of Molecular Biology, together with the mechanistic insights from Paul Boyer and the pioneering work of others, represented a collaborative tour de force which led to the award of a Nobel prize in 1978. The ATP synthase molecule is much larger than most enzymes (it is clearly visible in electron micrographs of the inner mitochondrial membrane). The model right, illustrates the build up of a proton gradient, either side of a membrane: this reaches a critical threshold, at which point the base rotates as shown. This is coupled to the phosphorylation of ADP: the flow of electrons along the electron transfer chain, arranged on the inner mitochondrial membrane promotes the formation of a proton gradient that provides the energy for ATP production.
The work of John Walker's lab at the Laboratory of Molecular Biology, together with the mechanistic insights from Paul Boyer and the pioneering work of others, represented a collaborative tour de force which led to the award of a Nobel prize in 1978. The ATP synthase molecule is much larger than most enzymes (it is clearly visible in electron micrographs of the inner mitochondrial membrane). The model right, illustrates the build up of a proton gradient, either side of a membrane: this reaches a critical threshold, at which point the base rotates as shown. This is coupled to the phosphorylation of ADP: the flow of electrons along the electron transfer chain, arranged on the inner mitochondrial membrane promotes the formation of a proton gradient that provides the energy for ATP production.
A brief word on ATP is required here. Fritz Lipmann had identified the importance of this molecule in providing cellular energy, but why is it so special? The hydrolysis of the terminal (gamma) phosphate converting ATP to ADP and inorganic phosphate (the reverse of the ATP synthase reaction), yields approximately 30kJ/mol (the hydrolysis of 2 phosphates, yields around 45kJ/mol and AMP) of energy. The exergonic nature of this hydrolysis is in part due to the favourable resonance stabilisation of the free phosphate compared with ATP, together with the relative instability of a string of negative charges in ATP. Interestingly GTP (recall nucleic acids are built from ATP, GTP,TTP (or UTP) and CTP), is the molecule that provides the energy for protein synthesis: it has the same energetic characteristics as ATP and both are synthesised de novo and by recovery from fragments (a process called the salvage pathway).
The importance of ATP synthase and ATP cannot be overstated in Biology, and the development of the chemiosmotic theory that rationalises the chemistry and physics behind this process is as fascinating as the scientists involved.

Peter Mitchell graduated from Cambridge over 60 years ago: in fact his first project proved unsatisfactory, but in 1950 he finally earned his PhD stripes. ATP was first discovered in 1929, and in the period 1940 to 1960 the compound was synthesised chemically by Todd; a few years earlier, Lipmann had recognised its potential for harnessing biochemical energy, but despite a considerable effort attempts to rationalise the data, the evidence for its biological synthesis remained unsatisfactory. From the insights of a great British Biochemist RJP Williams, however, Mitchell was able to propose how harnessing the energy in a proton gradient, established acoss biological membranes, could be coupled to the synthesis of ATP. That is, a proton-motive-force drives structural changes in the ATP synthase: this was corroborated by the work of the famous enzymologist Paul Boyer from Los Angeles and our own Sir John Walker (below left, at Cambridge).
The union of ADP and inorganic phosphate is brought about (against an adverse energy barrier) through the coupling of a conformational change in a subunit of the ATP synthase and the movement of protons across a membrane. At the time, this idea was radical, so much so that Mitchell was forced to build his own private research facility on Bodmin Moore (a favourite location for Gothic mysteries, and the legendary home of King Arthur and the sword excalibur). The reclusive Mitchell (along with his brother) resorted to publishing his own ideas. First, it was published as a pamphlet, but two years later, he followed up with several small volumes called “little grey books” named after their rather unexciting covers. Just like change, investigative research is constant, and in the early 1970s, his theory gained significant acceptance among his peers and the scientific community in general. Meanwhile, John Walker was developing the exacting purification methods in order to obtain the mg quantities necessary for detailed structural studies:. With a structure in hand predictions can be tested, although the scale of this task was a major challenge and the confirmation came slowly.
 The work of John Walker's lab at the Laboratory of Molecular Biology, together with the mechanistic insights from Paul Boyer and the pioneering work of others, represented a collaborative tour de force which led to the award of a Nobel prize in 1978. The ATP synthase molecule is much larger than most enzymes (it is clearly visible in electron micrographs of the inner mitochondrial membrane). The model right, illustrates the build up of a proton gradient, either side of a membrane: this reaches a critical threshold, at which point the base rotates as shown. This is coupled to the phosphorylation of ADP: the flow of electrons along the electron transfer chain, arranged on the inner mitochondrial membrane promotes the formation of a proton gradient that provides the energy for ATP production.
The work of John Walker's lab at the Laboratory of Molecular Biology, together with the mechanistic insights from Paul Boyer and the pioneering work of others, represented a collaborative tour de force which led to the award of a Nobel prize in 1978. The ATP synthase molecule is much larger than most enzymes (it is clearly visible in electron micrographs of the inner mitochondrial membrane). The model right, illustrates the build up of a proton gradient, either side of a membrane: this reaches a critical threshold, at which point the base rotates as shown. This is coupled to the phosphorylation of ADP: the flow of electrons along the electron transfer chain, arranged on the inner mitochondrial membrane promotes the formation of a proton gradient that provides the energy for ATP production.A brief word on ATP is required here. Fritz Lipmann had identified the importance of this molecule in providing cellular energy, but why is it so special? The hydrolysis of the terminal (gamma) phosphate converting ATP to ADP and inorganic phosphate (the reverse of the ATP synthase reaction), yields approximately 30kJ/mol (the hydrolysis of 2 phosphates, yields around 45kJ/mol and AMP) of energy. The exergonic nature of this hydrolysis is in part due to the favourable resonance stabilisation of the free phosphate compared with ATP, together with the relative instability of a string of negative charges in ATP. Interestingly GTP (recall nucleic acids are built from ATP, GTP,TTP (or UTP) and CTP), is the molecule that provides the energy for protein synthesis: it has the same energetic characteristics as ATP and both are synthesised de novo and by recovery from fragments (a process called the salvage pathway).
The importance of ATP synthase and ATP cannot be overstated in Biology, and the development of the chemiosmotic theory that rationalises the chemistry and physics behind this process is as fascinating as the scientists involved.


I have been telling my A2 Biology group at the UTC this week about this marvellous little biological motor! I shall direct them to this blog :)
ReplyDeleteExcellent, thanks. I was talking to them about the similarities between Nitroglycerin and ATP. Both pack a punch when the charges are released!
Deleteprof premraj pushpakaran writes -- 2018 marks the 100th birth year of Paul Delos Boyer!!!
ReplyDeleteprof premraj pushpakaran writes -- 2018 marks the 100th birth year of Paul Delos Boyer!!!
ReplyDelete