August
is usually the hottest month in the Northern Hemisphere and a time when
our skin can be exposed to risk from both high temperatures and ultra
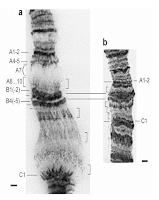
violet irradiation, it seemed timely then to pick a class of molecule that helps us deal with "heat shock". The "puffing" of chromatin (compare a and b left from Michael Ashburner's work) is associated with the "switching on " of heat shock genes. This is a Biological phenomenon that was described over 50 years ago in (mainly) Drosophila, and later captured the interest of an old friend of mine who sadly passed away last month, Tony (AR) Clarke.
It is also a molecule (well a class of molecule really) whose structure
and function has been the subject of Biochemical and Biophysical
investigation in a number of other UK labs, including Laurence Pearl's lab at Sussex and at the Martinsried Max Planck Institute in the laboratory of Ulrich Hartl. And since the publication of the first electron microscopy images and subsequent crystal structures, the number of molecular studies of this class of proteins has increased significantly.
In an earlier post, I discussed the fundamental significance of Christian B Anfinsen's work on the relationship between primary and tertiary structure in proteins. You may also recall (or take a look?) the caveats emerging, that suggest a given primary structure does not always give rise to a unique tertiary structure. However, there is another important mechanism involved in the translation (I use this word in its general sense) of protein sequence into three dimensional structure and function, and it has its origins in the work on "heat shock". It is now time to introduce the concept of the "molecular chaperone". The word chaperone was formerly used in the context of social norms in relation to the "appropriateness" of a single women to be "out" in public. A chaperone would, in this context, be typically an older woman (maybe an aunt or family friend) who would accompany a young, single woman to social events or visits to Fortnum and Masons, for a little retail therapy. The word is derived from old French, meaning a cover or hood (un chapeau: a hat, used to be in the first division of French vocabulary lists when I was at school). In some ways these two function: a covering and a protector, describe the properties of chaperone proteins very nicely. These molecules provide a safe haven for some newly synthesised proteins (see the image from Ulrich Hartl's web site above), where the formation of tertiary structure requires a "leg up". I think this wiki site provides a nice, simple introduction to chaperone proteins. More detailed open access reviews include this one from Laurence Pearl in which he discusses Hsp90 structure and function in some detail, and refers extensively to the concept of "client" proteins, serviced by this particular molecular chaperone. I should also add that molecular chaperones are also referred to as chaperonins, but I think we can handle that!
Before I begin. Think for a moment about how you might "design" a molecular chaperone. This could form the basis of a nice "brainstorming" tutorial? Does every protein coding gene require its own chaperone to assist in folding, or perhaps more accurately prevent inappropriate aggregation: the primary role of chaperones in heat shock? How diverse are proteins in terms of molecular volume? Could a series of molecular "cages" say, accommodate small, medium and large proteins? How would a chaperone "attract" an unfolded, or "wayward" polypeptide chain and provide it with a safe haven? What is it that the safe haven could offer that the cytosol does not? How does the chaperone know when a protein is folded correctly? And when to let it go? How long does it take a non-Anfinsen polypeptide to emerge from a chaperone? What proportion of a cellular proteome is chaperone dependent? These and other considerations must be addressed in "designing" such a protein. I think this is a good undergraduate tutorial challenge.
The structure on the left is a complex between the cylindrical GroEL and the cap protein, GroES, one of the first chaperones to be described in biochemical detail. The "channel" of GroEL is approximately 5nm, which can accommodate some, but not all polypeptides. However, if we consider its modus operandi, we can begin to appreciate that the mouth of the chaperone only needs to accommodate the unfolded (or partially folded) polypeptide chain. It is therefore an elongated cylinder and its interior attenuates the hydrophobic effect that drives many proteins to fold in the Anfinsen way. (The dimensions are given in the diagram below in Angstrom units: divide by 10 to covert to nm).
 Clearly, every polypeptide chain is different, and yet there are only a handful of chaperones (see Laurence Pearl's review for a discussion of co-chaperones and clients), chaperones must have been selected to be shared between several thousand unique ORFs (in E.coli): there must be some general interactions that offer an alternative to those available in the cellular milieu. Remember in the Anfinsen model a polypeptide chain is effectively "immersed" in water and immediately self-organises through backbone and side chain interactions,
driven by the partitioning of polar and non-polar groups. The fine
tuning of the three dimensional structure comes through the directional stabilisation of hydrogen bonds, ionic interactions and equilibration of hydrophobic interactions to achieve (mostly) a unique, minimum free energy state.
Clearly, every polypeptide chain is different, and yet there are only a handful of chaperones (see Laurence Pearl's review for a discussion of co-chaperones and clients), chaperones must have been selected to be shared between several thousand unique ORFs (in E.coli): there must be some general interactions that offer an alternative to those available in the cellular milieu. Remember in the Anfinsen model a polypeptide chain is effectively "immersed" in water and immediately self-organises through backbone and side chain interactions,
driven by the partitioning of polar and non-polar groups. The fine
tuning of the three dimensional structure comes through the directional stabilisation of hydrogen bonds, ionic interactions and equilibration of hydrophobic interactions to achieve (mostly) a unique, minimum free energy state.
For non-Anfinsen polypeptides, a little help is needed to keep the polypeptide chain along the "straight and narrow", by closing down alternative, non-productive folding routes and inappropriate inter-molecular liaisons (such as those arising through aggregation). The diagram below provides an indication of the stages in in chaperone mediated protein folding, which as you can see is energy requiring. It is therefore important to recognise that this process is a necessary evil, rather than a luxury item, since the correct assembly of several complex protein functions requires assistance. There must therefore be a co-dependence of the evolution of such proteins and their chaperone partners. The client: chaperone relationship, is itself the subject of much interest in the literature and is being exploited in the search for new therapeutic molecules (see work from the Pearl lab, for example).
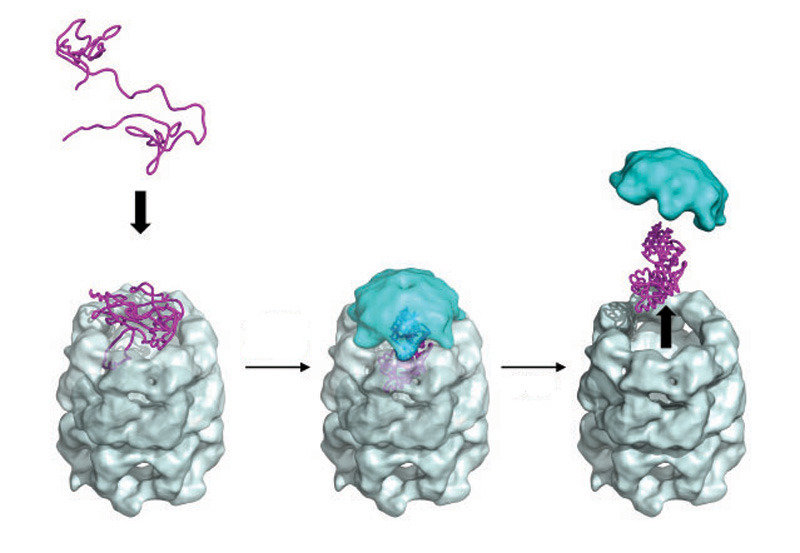
 The figure on the LHS, is taken from a review by Bigotti and Clarke and described the stages in the "capture" of a polypeptide substrate by the GroE system. Gro EL is in grey and blue, with the polypeptide sandwiched between the "mouthparts" which pull in the polypetide substrate in response to ATP addition. In this form, GroES now
binds rapidly with high affinity and the polypeptide enters the cavity
where folding takes place. The release of the protein follows two possible paths in vivo. In the "standard" sense, the folded protein is released for duty. However, unfolded polypeptides can also be sequestered for degradation via the proteosome in a similar, but opposite way. It must be remembered that a cellular proteome represents a steady state of synthesis and turn-over and similarly, all enzymatic reactions are in principle reversible: directionality is imposed by the downstream, biochemical demands.
The figure on the LHS, is taken from a review by Bigotti and Clarke and described the stages in the "capture" of a polypeptide substrate by the GroE system. Gro EL is in grey and blue, with the polypeptide sandwiched between the "mouthparts" which pull in the polypetide substrate in response to ATP addition. In this form, GroES now
binds rapidly with high affinity and the polypeptide enters the cavity
where folding takes place. The release of the protein follows two possible paths in vivo. In the "standard" sense, the folded protein is released for duty. However, unfolded polypeptides can also be sequestered for degradation via the proteosome in a similar, but opposite way. It must be remembered that a cellular proteome represents a steady state of synthesis and turn-over and similarly, all enzymatic reactions are in principle reversible: directionality is imposed by the downstream, biochemical demands.
Returning to the idea of a brainstorming turorial, I always think it is a good exercise to ask students to work out the phenomenal kinetic challenge of cell division, starting from an unreplicated and unexpressed genome comprising 3 000 genes and sufficient glucose to t urn a single colony inoculum into say 5g cells in a litre culture overnight! It is then that you begin to appreciate just how successful evolution has been! What I have never done is to compare the time taken for a an Anfinsen and a non-Anfinsen protein of the same size to "report for duty". Time to read the literature more thoroughly, I think.
In an earlier post, I discussed the fundamental significance of Christian B Anfinsen's work on the relationship between primary and tertiary structure in proteins. You may also recall (or take a look?) the caveats emerging, that suggest a given primary structure does not always give rise to a unique tertiary structure. However, there is another important mechanism involved in the translation (I use this word in its general sense) of protein sequence into three dimensional structure and function, and it has its origins in the work on "heat shock". It is now time to introduce the concept of the "molecular chaperone". The word chaperone was formerly used in the context of social norms in relation to the "appropriateness" of a single women to be "out" in public. A chaperone would, in this context, be typically an older woman (maybe an aunt or family friend) who would accompany a young, single woman to social events or visits to Fortnum and Masons, for a little retail therapy. The word is derived from old French, meaning a cover or hood (un chapeau: a hat, used to be in the first division of French vocabulary lists when I was at school). In some ways these two function: a covering and a protector, describe the properties of chaperone proteins very nicely. These molecules provide a safe haven for some newly synthesised proteins (see the image from Ulrich Hartl's web site above), where the formation of tertiary structure requires a "leg up". I think this wiki site provides a nice, simple introduction to chaperone proteins. More detailed open access reviews include this one from Laurence Pearl in which he discusses Hsp90 structure and function in some detail, and refers extensively to the concept of "client" proteins, serviced by this particular molecular chaperone. I should also add that molecular chaperones are also referred to as chaperonins, but I think we can handle that!
Before I begin. Think for a moment about how you might "design" a molecular chaperone. This could form the basis of a nice "brainstorming" tutorial? Does every protein coding gene require its own chaperone to assist in folding, or perhaps more accurately prevent inappropriate aggregation: the primary role of chaperones in heat shock? How diverse are proteins in terms of molecular volume? Could a series of molecular "cages" say, accommodate small, medium and large proteins? How would a chaperone "attract" an unfolded, or "wayward" polypeptide chain and provide it with a safe haven? What is it that the safe haven could offer that the cytosol does not? How does the chaperone know when a protein is folded correctly? And when to let it go? How long does it take a non-Anfinsen polypeptide to emerge from a chaperone? What proportion of a cellular proteome is chaperone dependent? These and other considerations must be addressed in "designing" such a protein. I think this is a good undergraduate tutorial challenge.
The structure on the left is a complex between the cylindrical GroEL and the cap protein, GroES, one of the first chaperones to be described in biochemical detail. The "channel" of GroEL is approximately 5nm, which can accommodate some, but not all polypeptides. However, if we consider its modus operandi, we can begin to appreciate that the mouth of the chaperone only needs to accommodate the unfolded (or partially folded) polypeptide chain. It is therefore an elongated cylinder and its interior attenuates the hydrophobic effect that drives many proteins to fold in the Anfinsen way. (The dimensions are given in the diagram below in Angstrom units: divide by 10 to covert to nm).
 Clearly, every polypeptide chain is different, and yet there are only a handful of chaperones (see Laurence Pearl's review for a discussion of co-chaperones and clients), chaperones must have been selected to be shared between several thousand unique ORFs (in E.coli): there must be some general interactions that offer an alternative to those available in the cellular milieu. Remember in the Anfinsen model a polypeptide chain is effectively "immersed" in water and immediately self-organises through backbone and side chain interactions,
driven by the partitioning of polar and non-polar groups. The fine
tuning of the three dimensional structure comes through the directional stabilisation of hydrogen bonds, ionic interactions and equilibration of hydrophobic interactions to achieve (mostly) a unique, minimum free energy state.
Clearly, every polypeptide chain is different, and yet there are only a handful of chaperones (see Laurence Pearl's review for a discussion of co-chaperones and clients), chaperones must have been selected to be shared between several thousand unique ORFs (in E.coli): there must be some general interactions that offer an alternative to those available in the cellular milieu. Remember in the Anfinsen model a polypeptide chain is effectively "immersed" in water and immediately self-organises through backbone and side chain interactions,
driven by the partitioning of polar and non-polar groups. The fine
tuning of the three dimensional structure comes through the directional stabilisation of hydrogen bonds, ionic interactions and equilibration of hydrophobic interactions to achieve (mostly) a unique, minimum free energy state. For non-Anfinsen polypeptides, a little help is needed to keep the polypeptide chain along the "straight and narrow", by closing down alternative, non-productive folding routes and inappropriate inter-molecular liaisons (such as those arising through aggregation). The diagram below provides an indication of the stages in in chaperone mediated protein folding, which as you can see is energy requiring. It is therefore important to recognise that this process is a necessary evil, rather than a luxury item, since the correct assembly of several complex protein functions requires assistance. There must therefore be a co-dependence of the evolution of such proteins and their chaperone partners. The client: chaperone relationship, is itself the subject of much interest in the literature and is being exploited in the search for new therapeutic molecules (see work from the Pearl lab, for example).
 The figure on the LHS, is taken from a review by Bigotti and Clarke and described the stages in the "capture" of a polypeptide substrate by the GroE system. Gro EL is in grey and blue, with the polypeptide sandwiched between the "mouthparts" which pull in the polypetide substrate in response to ATP addition. In this form, GroES now
binds rapidly with high affinity and the polypeptide enters the cavity
where folding takes place. The release of the protein follows two possible paths in vivo. In the "standard" sense, the folded protein is released for duty. However, unfolded polypeptides can also be sequestered for degradation via the proteosome in a similar, but opposite way. It must be remembered that a cellular proteome represents a steady state of synthesis and turn-over and similarly, all enzymatic reactions are in principle reversible: directionality is imposed by the downstream, biochemical demands.
The figure on the LHS, is taken from a review by Bigotti and Clarke and described the stages in the "capture" of a polypeptide substrate by the GroE system. Gro EL is in grey and blue, with the polypeptide sandwiched between the "mouthparts" which pull in the polypetide substrate in response to ATP addition. In this form, GroES now
binds rapidly with high affinity and the polypeptide enters the cavity
where folding takes place. The release of the protein follows two possible paths in vivo. In the "standard" sense, the folded protein is released for duty. However, unfolded polypeptides can also be sequestered for degradation via the proteosome in a similar, but opposite way. It must be remembered that a cellular proteome represents a steady state of synthesis and turn-over and similarly, all enzymatic reactions are in principle reversible: directionality is imposed by the downstream, biochemical demands.Returning to the idea of a brainstorming turorial, I always think it is a good exercise to ask students to work out the phenomenal kinetic challenge of cell division, starting from an unreplicated and unexpressed genome comprising 3 000 genes and sufficient glucose to t urn a single colony inoculum into say 5g cells in a litre culture overnight! It is then that you begin to appreciate just how successful evolution has been! What I have never done is to compare the time taken for a an Anfinsen and a non-Anfinsen protein of the same size to "report for duty". Time to read the literature more thoroughly, I think.

No comments:
Post a Comment