 When
I think of car tyres, I don't automatically think of Biological
molecules, but latex is the product of an unusual biosynthetic pathway
in the rubber plant Hevea brasiliensis, the Pará rubber tree, sharinga
tree, or, most commonly, simply, the rubber tree (shown on the LHS).
Isopentenyl pyrophosphate is the monomeric precursor (above RHS) for the
biosynthesis of the rubber polymer. The industrial process for
generating synthetic rubber (SR) follows a quite different route, but
the polymeric product is essentially the same. In addition, each natural
rubber polymer (NR) varies in "formulation" since the rubber particles
are associated with lipids and proteins that are species and
cell-specific. I won't have time to discuss this here, but you can read
more about this fascinating natural product here). The polymeric backbone is shown below.
When
I think of car tyres, I don't automatically think of Biological
molecules, but latex is the product of an unusual biosynthetic pathway
in the rubber plant Hevea brasiliensis, the Pará rubber tree, sharinga
tree, or, most commonly, simply, the rubber tree (shown on the LHS).
Isopentenyl pyrophosphate is the monomeric precursor (above RHS) for the
biosynthesis of the rubber polymer. The industrial process for
generating synthetic rubber (SR) follows a quite different route, but
the polymeric product is essentially the same. In addition, each natural
rubber polymer (NR) varies in "formulation" since the rubber particles
are associated with lipids and proteins that are species and
cell-specific. I won't have time to discuss this here, but you can read
more about this fascinating natural product here). The polymeric backbone is shown below.The starting point for rubber biosynthesis is IPP (half of the molecule of the month: the second half is its polymerised form, rubber). The general pathway for rubber biosynthesis from IPP is shown below). You can read a recent genomic analysis of the pathways here.
What most interests me about rubber is the idea that cells can make such apparently "Inert", flexible insulating molecules which are in some ways the opposite of our own bones or a snail's shell.
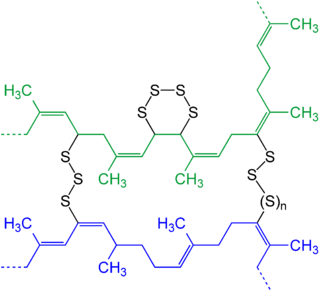
The properties of rubber have led to a major industry and the development of industrial processes that convert NR into a useful product, such as in the manufacture of car and bicycle tyres. The process you will all have heard of is vulcanisation. Essentially, this proces adds sulphur to rubber (although other compounds may be added), leading to a more robust, stiffer form of the polymer (see RHS). Vulcanisation takes its name from Vulcan, the Roman god of fire and was patented most successfully by an American amateur chemist, Charles Goodyear (the English industrial chemist Thomas Hancock also filed a patent on the method of vulcanisation pioneer, but this is another story). Like natural collagen, which forms spontaneous triple helices followed by age related cross links, rubber can be converted into a whole range of formulations in order to meet the requirements of a particular application. I am also reminded of disulphide linkages that link the two chains of insulin together, or the heavy and light chains of antibody molecules. The drive to find ways of modifying the elastic and mechanical properties of rubber have led to a solution that Nature also adopts (or should I say adopted, a little earlier). If you find this area interesting, you may like to look up elastin, a polypeptide (family) with a repeated sequence of the type: GVPGXG (where X cannot be P).
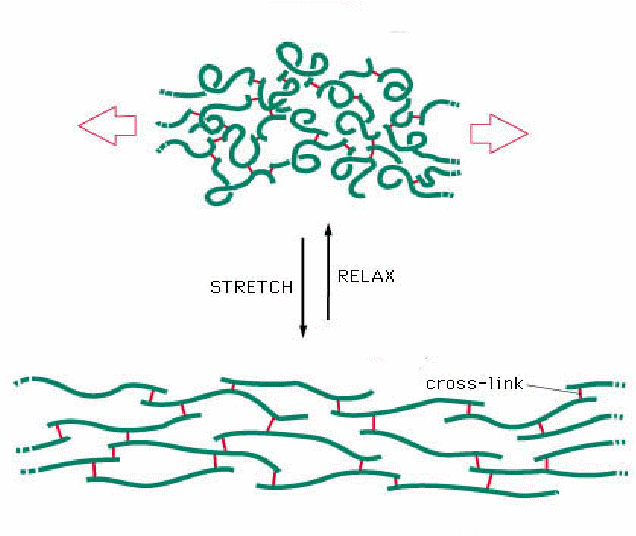 As a final point for discussion in a tutorial, one of the most familiar properties of rubber polymers is their elasticity: i.e. rubber can be reversibly stretched. When you stretch an elastic band it gets hot, when you let go, the rubber cools. The "ground state", or unstretched polymer chains, when relaxed are disordered (recall the second law of thermodynamics), however as they are stretched, the polymer chains align, becoming more organised, relative to the unstretched, ground state, thereby reducing entropy. As a result, heat is released. Stretching requires a physical input of energy and is a relatively slow process: snapping back to the ground state, on the other hand, is spontaneous. In kinetic terms, this provides a great example of the "power" of entropy, oh and it also provides food for kinetic thought too! Finally, the the sustainable and reversible elasticity of rubber is improved by vulcanisation: can this help us understand and explain the role of disulphide bonds in protein structure? These phenomena are encountered frequently when
biophysicists explore the thermodynamics and kinetics of protein
folding. I think a few elastic bands are a simple way to seed a tutorial
on the kinetics and thermodynamics of macromolecular folding? A final point for discussion could be the observation that rubber bands typically "perish" over a year or so. Why? This maybe more useful in discussions of nucleic acid polymers? Anyway, I hope this Molecule of the Month illustrates the Venn Diagram between Life Sciences. Chemistry and Physics!
As a final point for discussion in a tutorial, one of the most familiar properties of rubber polymers is their elasticity: i.e. rubber can be reversibly stretched. When you stretch an elastic band it gets hot, when you let go, the rubber cools. The "ground state", or unstretched polymer chains, when relaxed are disordered (recall the second law of thermodynamics), however as they are stretched, the polymer chains align, becoming more organised, relative to the unstretched, ground state, thereby reducing entropy. As a result, heat is released. Stretching requires a physical input of energy and is a relatively slow process: snapping back to the ground state, on the other hand, is spontaneous. In kinetic terms, this provides a great example of the "power" of entropy, oh and it also provides food for kinetic thought too! Finally, the the sustainable and reversible elasticity of rubber is improved by vulcanisation: can this help us understand and explain the role of disulphide bonds in protein structure? These phenomena are encountered frequently when
biophysicists explore the thermodynamics and kinetics of protein
folding. I think a few elastic bands are a simple way to seed a tutorial
on the kinetics and thermodynamics of macromolecular folding? A final point for discussion could be the observation that rubber bands typically "perish" over a year or so. Why? This maybe more useful in discussions of nucleic acid polymers? Anyway, I hope this Molecule of the Month illustrates the Venn Diagram between Life Sciences. Chemistry and Physics!

No comments:
Post a Comment