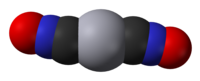 One dictionary definition of the verb to fulminate is "to explode violently". A second, more chemical definition would be "to react a metal with nitric acid and ethanol". The result of the latter, as discovered by Edward Charles Howard in 1824, is a highly explosive compound comprising a metal and a fulminate ion
One dictionary definition of the verb to fulminate is "to explode violently". A second, more chemical definition would be "to react a metal with nitric acid and ethanol". The result of the latter, as discovered by Edward Charles Howard in 1824, is a highly explosive compound comprising a metal and a fulminate ion which is also shown top left as mercury fulminate in a space filling format. In fact mercury and silver are the best known of the fulminates. The reason for discussing them here is partly because it is Nobel Prize month, and this time last year I discussed dynamite and nitroglycerin. This year I am attracted to these molecules because of what they can also teach us about electrons, bonding and energy. [If you didn't know, Alfred Nobel made his fortune from explosives!].
 Chemistry at the turn of the 19th century was largely empirical. That is, most experiments were look-see, and relatively few scientists carried out systematic investigations. There were of course some notable exceptions: but in trying to harness the power of chemistry during Queen Victoria's reign in England, was not dissimilar to the attempts to harness the power of molecular biology in the early days of the Biotechnology industry. In other ways the knowledge base was inadequate. In fact Mendeleev's first Periodic Table was published in 1869, about 40 years after the start of the Industrial Revolution and the birth of the Chemical Industry! So when Edward Charles Howard (above) added mercury to nitric acid, followed by a dash of ethanol, mercury fulminate was "born". [Just think of the Health and Safety consequences for proposing this as a first year chemistry practical!]. Silver fulminate, prepared in the same way is even more unstable and can actually explode under its own weight, and under water!
Chemistry at the turn of the 19th century was largely empirical. That is, most experiments were look-see, and relatively few scientists carried out systematic investigations. There were of course some notable exceptions: but in trying to harness the power of chemistry during Queen Victoria's reign in England, was not dissimilar to the attempts to harness the power of molecular biology in the early days of the Biotechnology industry. In other ways the knowledge base was inadequate. In fact Mendeleev's first Periodic Table was published in 1869, about 40 years after the start of the Industrial Revolution and the birth of the Chemical Industry! So when Edward Charles Howard (above) added mercury to nitric acid, followed by a dash of ethanol, mercury fulminate was "born". [Just think of the Health and Safety consequences for proposing this as a first year chemistry practical!]. Silver fulminate, prepared in the same way is even more unstable and can actually explode under its own weight, and under water! There is another twist to the fulminate story. The chemists among you will have recognised that the formulae for silver cyanate (AgOCN) and and silver fulminate (AgCNO) are technically equivalent. In the 1820s, these observations led to a huge debate betwee one of history's most illustrious chemists, Justus Liebig, who discovered silver fulminate (Ag-CNO) and Friedrich
Wšhler, who discovered silver cyanate (Ag-OCN). This was only resolved when Jšns Jakob Berzelius came up with
the concept of isomers. The tension in the fulminate molecule seemed to have rubbed off on Liebig and
Wšhler! It was also Alfred Nobel who had the last laugh: he patented the use of mercury fulminate in his explosives and therby generated even more wealth. Finally, although the fulminic acid molecule looks very simple, its structure was only determined about 10 years ago! I think this is a suitable choice for the Nobel month of October and I wonder what explosions will emerge next week when the Nobel committee announce the results of their ruminations and deliberations.
There is another twist to the fulminate story. The chemists among you will have recognised that the formulae for silver cyanate (AgOCN) and and silver fulminate (AgCNO) are technically equivalent. In the 1820s, these observations led to a huge debate betwee one of history's most illustrious chemists, Justus Liebig, who discovered silver fulminate (Ag-CNO) and Friedrich
Wšhler, who discovered silver cyanate (Ag-OCN). This was only resolved when Jšns Jakob Berzelius came up with
the concept of isomers. The tension in the fulminate molecule seemed to have rubbed off on Liebig and
Wšhler! It was also Alfred Nobel who had the last laugh: he patented the use of mercury fulminate in his explosives and therby generated even more wealth. Finally, although the fulminic acid molecule looks very simple, its structure was only determined about 10 years ago! I think this is a suitable choice for the Nobel month of October and I wonder what explosions will emerge next week when the Nobel committee announce the results of their ruminations and deliberations.
No comments:
Post a Comment