 Some of the most enjoyable experiments last term involved the use of coloured solutions ranging from copper sulphate, for demonstrating the relationship between light absorbance and concentration to column chromatography. We also used milk to demonstrate the way in which salts such as ammonium sulphate and acids, such as acetic acid, could be used to fractionate proteins. Of course we also used ion exchange chromatography to separate proteins in milk on the basis of their surface charge. And of course, as you know milk is white.
Some of the most enjoyable experiments last term involved the use of coloured solutions ranging from copper sulphate, for demonstrating the relationship between light absorbance and concentration to column chromatography. We also used milk to demonstrate the way in which salts such as ammonium sulphate and acids, such as acetic acid, could be used to fractionate proteins. Of course we also used ion exchange chromatography to separate proteins in milk on the basis of their surface charge. And of course, as you know milk is white. So why are some metal salts colourless in aqueous solution and why are dyes (and the salts of some metals, such as copper sulphate) red, orange, green etc? Recall that we selected a specific wavelength of light to measure the relationship between the colour intensity of copper sulphate, this is because the specific arrangement of electrons in these compounds (or molecules) leads to the absorbance of certain wavelengths of light and the emitted light is now coloured. This relationship between light (photons) and matter is at the heart of biology in the form of photosynthesis. Refer to you class sheets for the structures of the dyes that we used: you will notice that they all contain aromatic rings and these ring structures contain "delocalised" electrons which often (but not always) give rise to the spectral characteristics of the molecule. Since the energy of a photon is inversely proportional to its wavelength. light of shorter wavelength has more energy than longer wavelengths and we can draw conclusions about the energy states of molecules from such experiments. This is again a major aspect of the Biophysics of photosynthesis.
The importance of many vitamins in Biology is a result of their role at the heart of many proteins including haemoglobin (which contains a form of porphyrin and iron, called haem) which transports oxygen around our cardiovascular system. Some enzymes are bright yellow in colour, owing to the presence of a flavin molecule, which facilitates the chemistry of catalysis that sometimes cannot be carried out by amino acids that make up the active site of the enzyme. You will also come to realise that energy in the form of the molecule ATP (adenosine triphosphate) is synthesised in the mitochondria following our metabolism of carbohydrates and fats via a series of complex events driven by electron flow between the haem components of a group of proteins called cytochromes. This process, which was first properly understood by the British
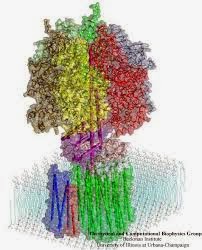
Biochemist Peter Mitchell, from his private laboratory on Bodmin Moor and around 30 years later, another British Biochemist, John Walker provided an important answer to the molecular framework of this phenomenon, when he determined the structure of one of the first molecular motors that couples electron and proton flow to ATP synthesis: the mitochondrial ATPase is shown on the right (or below). Isn't it an amazing molecule! Both scientists shared in separate Nobel Prizes. So understanding dyes can help us appreciate the way in which energy in the form of electrons within molecules can help us appreciate how Nature is able to mobilise energy from food and sunlight.

No comments:
Post a Comment