 One
of the essential tools of a hospital doctor is access to reliable and
safe anaesthetics. Today, Propofol is one of the most commonly used
general anaesthetics and forms subject of this post.
Its chemical structure is shown on the left and, as I think you can
see, it looks a little like the side chain of the proteogenic amino acid
tyrosine (Tyr or Y), or, as the chemical name indicates, the commonly
used disinfectant, phenol (which your grandparents would have called
carbolic acid, or simply carbolic). Today, you will find phenol in small
quantities in many products, largely owing to the low cost of
production and its general disinfectant properties. It is an ingredient
in many oral hygiene products, soaps, cosmetics and is a measurable
component of Islay Scotch Whisky! Now we are getting closer to the
anaesthetic properties of propofol! I should provide a definition and
the etymology of the word anaesthesia, before I go any further. The word
(sometimes without the second "a") was introduced by the famous American
Poet, Medic and Social reformer Oliver Wendell Holmes in 1846, to describe the inhibition of sensation: and is derived from the Greek αν-, an-, "without"
One
of the essential tools of a hospital doctor is access to reliable and
safe anaesthetics. Today, Propofol is one of the most commonly used
general anaesthetics and forms subject of this post.
Its chemical structure is shown on the left and, as I think you can
see, it looks a little like the side chain of the proteogenic amino acid
tyrosine (Tyr or Y), or, as the chemical name indicates, the commonly
used disinfectant, phenol (which your grandparents would have called
carbolic acid, or simply carbolic). Today, you will find phenol in small
quantities in many products, largely owing to the low cost of
production and its general disinfectant properties. It is an ingredient
in many oral hygiene products, soaps, cosmetics and is a measurable
component of Islay Scotch Whisky! Now we are getting closer to the
anaesthetic properties of propofol! I should provide a definition and
the etymology of the word anaesthesia, before I go any further. The word
(sometimes without the second "a") was introduced by the famous American
Poet, Medic and Social reformer Oliver Wendell Holmes in 1846, to describe the inhibition of sensation: and is derived from the Greek αν-, an-, "without";
and αἴσθησις,
aisthēsis,
"sensation").
 Phenol,
the "molecular core" of propofol, was first isolated in the early 19th
century in coal tar and later in petroleum. The dissociation of the
"12-o'clock" OH at high pH, makes phenol a weak acid: the benzene ring
draws electrons asymmetrically from the oxygen, facilitating release of
a proton and supporting (I assume) inter-molecular hydrogen bonding.
Hydrogen bonding, together with ring related van der Waal's
interactions, gives phenol its relatively high melting point (around 40
degrees C). It is a white crystalline solid (RHS) at room temperature,
but is hygroscopic (that volatile alcohol group again) and is often a
bit of a mess in a bottle in the summer! Getting back to propofol, my
question is really how can the basic skeleton of phenol in conjunction
with a symmetrical pair of isopropyl groups (-CH3-CH2-CH3), make most
humans fall fast asleep in a couple of minutes!
Phenol,
the "molecular core" of propofol, was first isolated in the early 19th
century in coal tar and later in petroleum. The dissociation of the
"12-o'clock" OH at high pH, makes phenol a weak acid: the benzene ring
draws electrons asymmetrically from the oxygen, facilitating release of
a proton and supporting (I assume) inter-molecular hydrogen bonding.
Hydrogen bonding, together with ring related van der Waal's
interactions, gives phenol its relatively high melting point (around 40
degrees C). It is a white crystalline solid (RHS) at room temperature,
but is hygroscopic (that volatile alcohol group again) and is often a
bit of a mess in a bottle in the summer! Getting back to propofol, my
question is really how can the basic skeleton of phenol in conjunction
with a symmetrical pair of isopropyl groups (-CH3-CH2-CH3), make most
humans fall fast asleep in a couple of minutes!
 The
first general anaesthetics to be introduced into modern medicine (I shall not discuss natural products here), follow the path of
the European Industrial Revolution and the birth of the Chemical
Industry. Joseph Priestly suggested the application of nitrous oxide
(laughing gas, N2O) at the turn of the 19th century and, following the
ancient uses of extracts of poppy, opium and morphine became (and
remain) potent analgaesics (without pain, as opposed to sensation), in
intensive therapies and surgery. But the most widely used anaesthetics
in the second half of the 19th century included ether (diethyl ether)
and chloroform. As you will appreciate, the rate of diffusion of these
gases is an important feature of their rapid action in creating
sleepiness and hallucinogenic effects.
The
first general anaesthetics to be introduced into modern medicine (I shall not discuss natural products here), follow the path of
the European Industrial Revolution and the birth of the Chemical
Industry. Joseph Priestly suggested the application of nitrous oxide
(laughing gas, N2O) at the turn of the 19th century and, following the
ancient uses of extracts of poppy, opium and morphine became (and
remain) potent analgaesics (without pain, as opposed to sensation), in
intensive therapies and surgery. But the most widely used anaesthetics
in the second half of the 19th century included ether (diethyl ether)
and chloroform. As you will appreciate, the rate of diffusion of these
gases is an important feature of their rapid action in creating
sleepiness and hallucinogenic effects.
 Propofol
was first discovered nearly 50 years ago at ICI, Cheshire (I assume
Macclesfield) and a patent filed under the number ICI 35868: clinical
trials were first reported in 1977. Unlike phenol, propofol is tricky to
formulate, since it is much less soluble in aqueous solution, and is
prepared as more of an emulsion. [I wont discuss formulation any further
here, but this is a critical aspect of the development of any drug, or
personal care product, as you can imagine, but one that is less
frequently discussed; look here if you are interested in formulation.] It has been shown that propofol binds to a range of proteins in vivo
including serum albumin, but more recently, its interaction with the
GABA receptor has been proposed as a key part of its effectiveness as an
anaesthetic (shown right is the proposed interaction between a derivative of propofol with a transmembrane region of the GABA-A receptor), from work by a US-UK collaboration between the laboratories
of Evers and Franks)
Propofol
was first discovered nearly 50 years ago at ICI, Cheshire (I assume
Macclesfield) and a patent filed under the number ICI 35868: clinical
trials were first reported in 1977. Unlike phenol, propofol is tricky to
formulate, since it is much less soluble in aqueous solution, and is
prepared as more of an emulsion. [I wont discuss formulation any further
here, but this is a critical aspect of the development of any drug, or
personal care product, as you can imagine, but one that is less
frequently discussed; look here if you are interested in formulation.] It has been shown that propofol binds to a range of proteins in vivo
including serum albumin, but more recently, its interaction with the
GABA receptor has been proposed as a key part of its effectiveness as an
anaesthetic (shown right is the proposed interaction between a derivative of propofol with a transmembrane region of the GABA-A receptor), from work by a US-UK collaboration between the laboratories
of Evers and Franks)
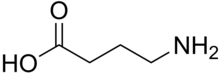 My
final point about general anaesthetics, such as propofol, relates to
their mechanism of action. Understanding the mode of action of these
compounds is a real challenge for Molecular Biologists. Clearly,
propofol and GABA (gamma amino butyric acid, shown left) are
unlikely to be competitive inhibitors! It seems clear to me that this
is an area of research that requires a "systems biology" approach
combined with high resolution structural biology (the work on mapping
the GABA-A site came from the use of tagged profolol analogues). The
interference with nerve transmission via chemical events and electrical
events (such as those orchestrated by our portfolio of ion channels),
appears to underpin the anaesthetic properties of molecules like
propofol, but rationalising, improving and making future anaesthetics
safer is such a key area of pharmacology, that I hope this molecule of
the month might stimulate further discussion with students who have a
general interest in the Molecular Life Sciences. Oh and Happy New Year
to all of my readers!
My
final point about general anaesthetics, such as propofol, relates to
their mechanism of action. Understanding the mode of action of these
compounds is a real challenge for Molecular Biologists. Clearly,
propofol and GABA (gamma amino butyric acid, shown left) are
unlikely to be competitive inhibitors! It seems clear to me that this
is an area of research that requires a "systems biology" approach
combined with high resolution structural biology (the work on mapping
the GABA-A site came from the use of tagged profolol analogues). The
interference with nerve transmission via chemical events and electrical
events (such as those orchestrated by our portfolio of ion channels),
appears to underpin the anaesthetic properties of molecules like
propofol, but rationalising, improving and making future anaesthetics
safer is such a key area of pharmacology, that I hope this molecule of
the month might stimulate further discussion with students who have a
general interest in the Molecular Life Sciences. Oh and Happy New Year
to all of my readers!
 The
first general anaesthetics to be introduced into modern medicine (I shall not discuss natural products here), follow the path of
the European Industrial Revolution and the birth of the Chemical
Industry. Joseph Priestly suggested the application of nitrous oxide
(laughing gas, N2O) at the turn of the 19th century and, following the
ancient uses of extracts of poppy, opium and morphine became (and
remain) potent analgaesics (without pain, as opposed to sensation), in
intensive therapies and surgery. But the most widely used anaesthetics
in the second half of the 19th century included ether (diethyl ether)
and chloroform. As you will appreciate, the rate of diffusion of these
gases is an important feature of their rapid action in creating
sleepiness and hallucinogenic effects.
The
first general anaesthetics to be introduced into modern medicine (I shall not discuss natural products here), follow the path of
the European Industrial Revolution and the birth of the Chemical
Industry. Joseph Priestly suggested the application of nitrous oxide
(laughing gas, N2O) at the turn of the 19th century and, following the
ancient uses of extracts of poppy, opium and morphine became (and
remain) potent analgaesics (without pain, as opposed to sensation), in
intensive therapies and surgery. But the most widely used anaesthetics
in the second half of the 19th century included ether (diethyl ether)
and chloroform. As you will appreciate, the rate of diffusion of these
gases is an important feature of their rapid action in creating
sleepiness and hallucinogenic effects. My
final point about general anaesthetics, such as propofol, relates to
their mechanism of action. Understanding the mode of action of these
compounds is a real challenge for Molecular Biologists. Clearly,
propofol and GABA (gamma amino butyric acid, shown left) are
unlikely to be competitive inhibitors! It seems clear to me that this
is an area of research that requires a "systems biology" approach
combined with high resolution structural biology (the work on mapping
the GABA-A site came from the use of tagged profolol analogues). The
interference with nerve transmission via chemical events and electrical
events (such as those orchestrated by our portfolio of ion channels),
appears to underpin the anaesthetic properties of molecules like
propofol, but rationalising, improving and making future anaesthetics
safer is such a key area of pharmacology, that I hope this molecule of
the month might stimulate further discussion with students who have a
general interest in the Molecular Life Sciences. Oh and Happy New Year
to all of my readers!
My
final point about general anaesthetics, such as propofol, relates to
their mechanism of action. Understanding the mode of action of these
compounds is a real challenge for Molecular Biologists. Clearly,
propofol and GABA (gamma amino butyric acid, shown left) are
unlikely to be competitive inhibitors! It seems clear to me that this
is an area of research that requires a "systems biology" approach
combined with high resolution structural biology (the work on mapping
the GABA-A site came from the use of tagged profolol analogues). The
interference with nerve transmission via chemical events and electrical
events (such as those orchestrated by our portfolio of ion channels),
appears to underpin the anaesthetic properties of molecules like
propofol, but rationalising, improving and making future anaesthetics
safer is such a key area of pharmacology, that I hope this molecule of
the month might stimulate further discussion with students who have a
general interest in the Molecular Life Sciences. Oh and Happy New Year
to all of my readers!